Spinal cord stimulation is regarded as a safe, effective, and reversible option for the management of chronic pain that has not responded to first-line interventions, such as medication or steroid injections. Chronic pain is a common condition experienced by many individuals during their lifetime. Lifetime prevalence rates of chronic pain conditions in the United States have been estimated to be as high as 30%. In fact, the Institute of Medicine has estimated that approximately 116 million people in the U.S. suffer from a chronic pain condition. Indeed, chronic pain is a worldwide problem. In 2006, 15 European countries were surveyed regarding their experience of chronic pain. Of the adults in the study, 19% of them were reported to have suffered from symptoms of pain lasting for more than six months. Further, this study revealed that over half of the individuals reporting having experienced a chronic pain condition were unable to work as a result of the pain. Moreover, almost 20% of those individuals indicated that they had lost their job as a result of their pain condition. Perhaps the most surprising findings from this study were that close to half of the individuals reporting having experienced symptoms of pain persisting for longer than six months were not receiving adequate care for the management of their pain episode, and only about 2% of them were actually being seen by a physician specializing in pain management.
Spinal Cord Stimulators are generally non-to minimally invasive and are conservative in nature.
Use the form on the right to request a call from our Patient Concierge Group.
Give us a call today at (919) 787-7246.[/color-box]
History of Spinal Cord Stimulation
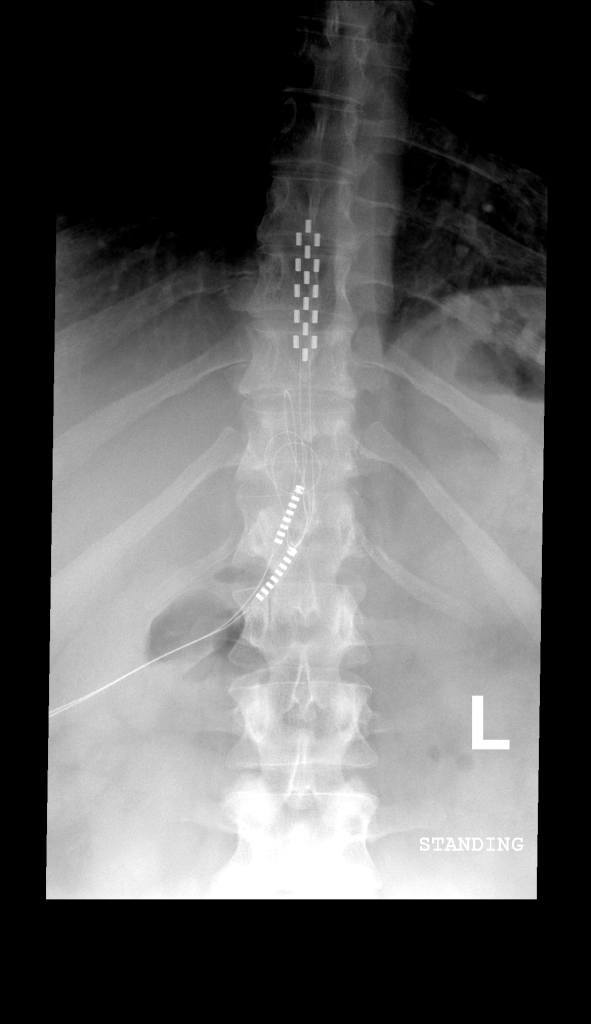
The origin of neurostimulation for the treatment of chronic pain arose following the emergence of the gate control theory. Originally proposed by Melzack and Wall in 1965, the gate control theory suggested that the dorsal horn of the spinal cord acted as a “gate” to sensation stimuli carried toward the spinal cord by the peripheral nerves. It was believed that signals of pain, which were carried to the spinal cord by the peripheral nervous system, could be interrupted by neurostimulation. Thus, it was postulated that implanting a stimulator device onto the dorsal column that would deliver electrical stimulation to the area would disrupt this pain sensation system. Indeed, in 1971, epidural spinal cord stimulation was first utilized successfully to treat a case of intractable pain.
Though it was originally developed on the foundation of the gate theory, the mechanisms of action for spinal cord stimulation’s analgesic properties are not fully known. There is a small body of literature that has attempted to explore this theory; however, their findings are inconclusive. It is likely that many factors acting in concert with one another can explain the pain relieving benefits of spinal cord stimulation on cases on intractable pain.
Types of Spinal Cord Stimulation and Procedure
Spinal cord stimulation involves the use of electrodes specifically designed for safe and effective implantation into the area near the spinal cord. The procedure itself involves placement of electrodes, along with a stimulation device. There are two types of spinal cord stimulator devices: transcutaneous and fully implantable. In terms of the transcutaneous spinal cord stimulator, the device is implanted subcutaneously (i.e., just beneath the surface of the skin). It is connected to an electrode that is positioned near the spinal cord. The transcutaneous device is the oldest spinal cord stimulation device still being used today. In fact, the transcutaneous device is preferred when high stimulation currents are required. Fully implantable pulse generators operate primarily off of battery power and are programed using an external device. Given this, it is necessary to replace the batteries in the fully implantable devices every two to five years. Patients report that they prefer the fully implantable device, to the transcutaneous device, because the device itself and the coil are not readily detected. Moreover, some patients have had difficulty with correct positioning of the transmitter coil (necessary for transmitting power to the implanted device and to transport pulse data) over the implanted device.